We collaborated with relevant product stakeholders, understood their business goals, the primary end-user profiles, and their goals as well as challenges. We leveraged the power of technology to digitise paper-based case report forms, and centralise data from various sources.
This resulted in a significant increase in data reconciliation and reporting efficiency, reduction in patient risks, and costs in conducting trials owing to manual tasks.
The Engagement
TRANSFORM
Summary of main achievments when we started
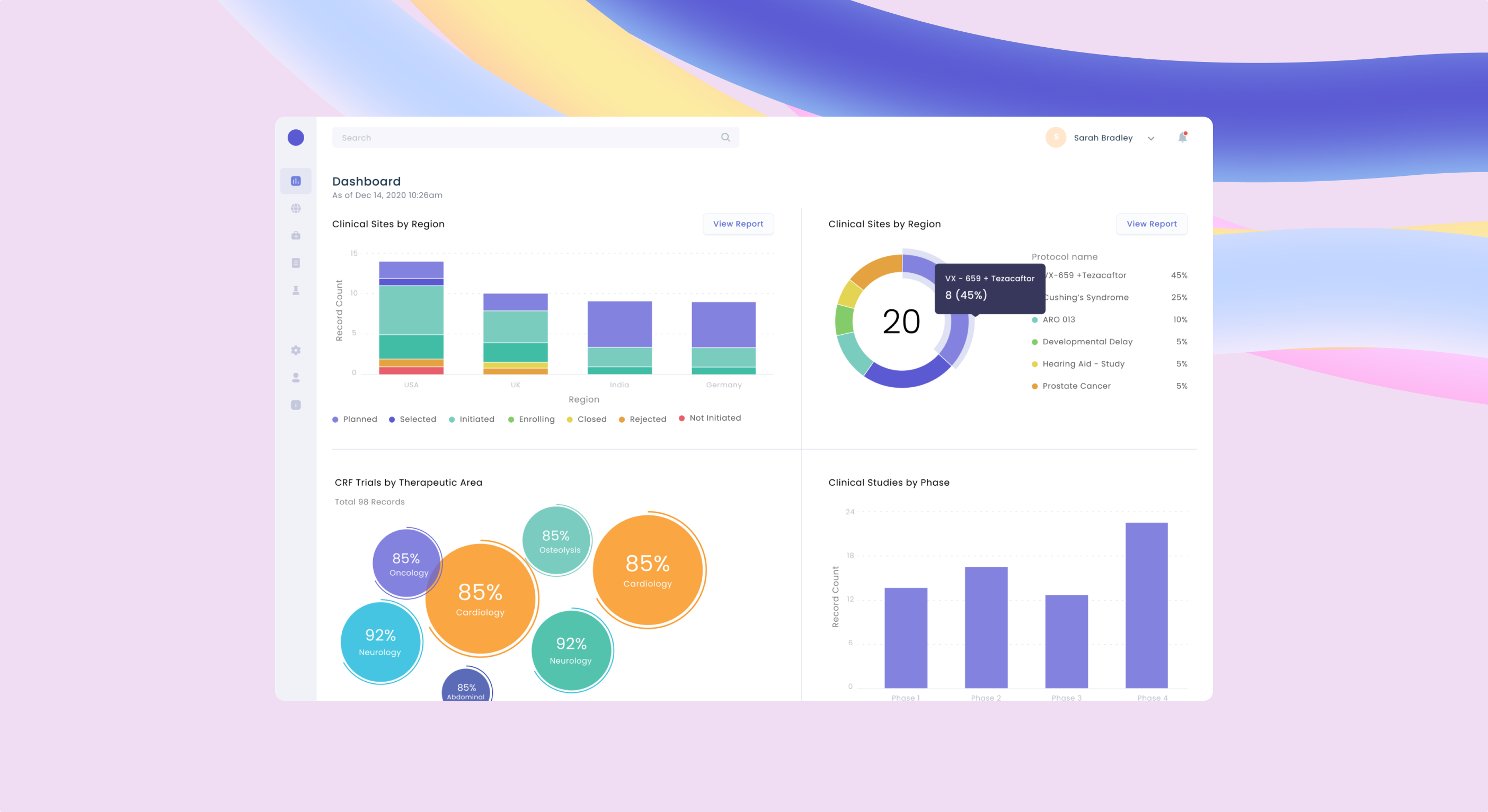
Centralised & real-time clinical trial data reporting.
Real time reporting & centralised data visualization on electronic case report from data
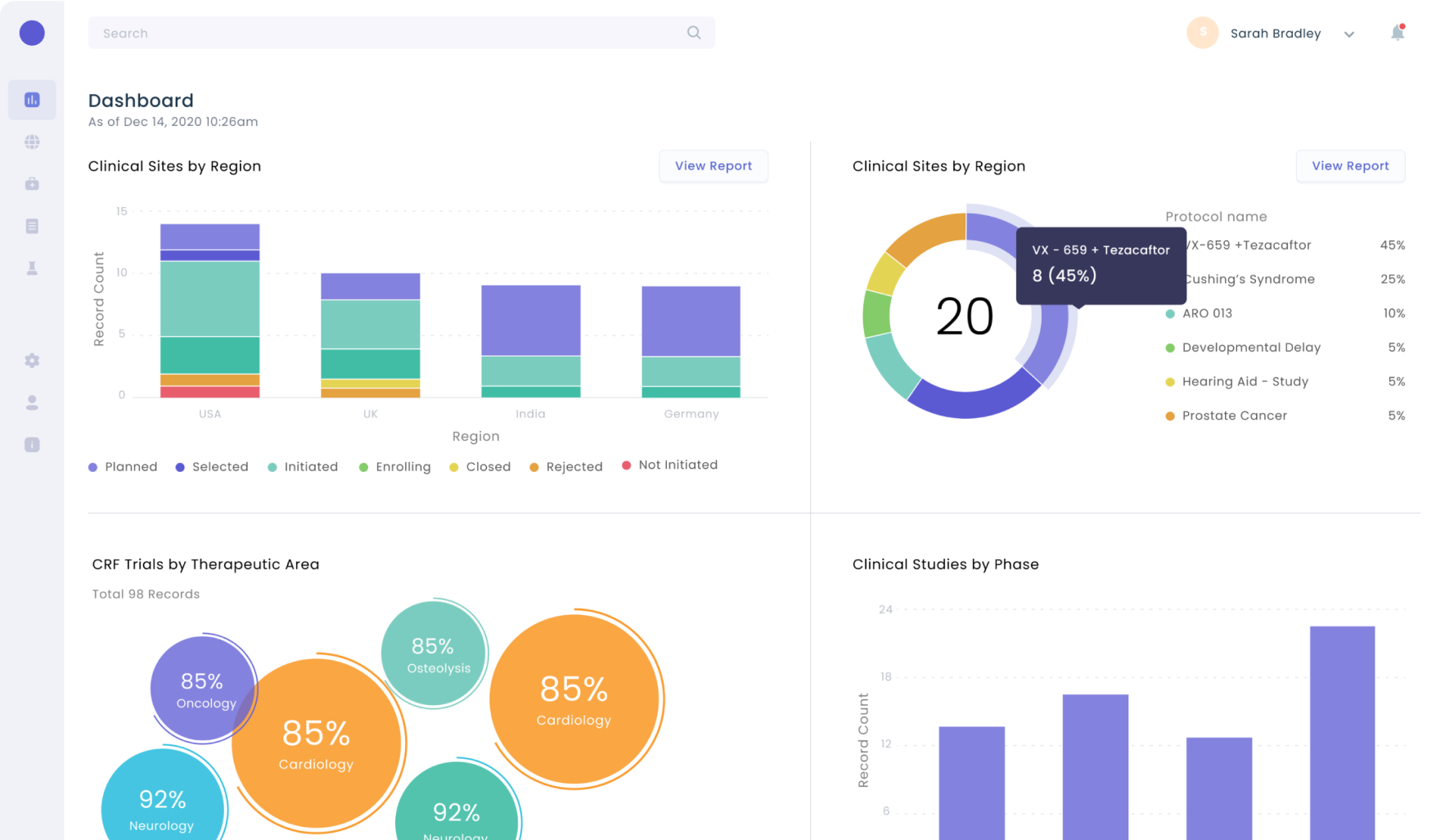
Data transparency and trustworthiness
Consent replaces the paper-based informed consent document. This provides real-time visibility and central tracking of recruitment and enrolment metrics across trial sites.
Digital Forms
End-users can drill-down to review the data and related details for quality checks.
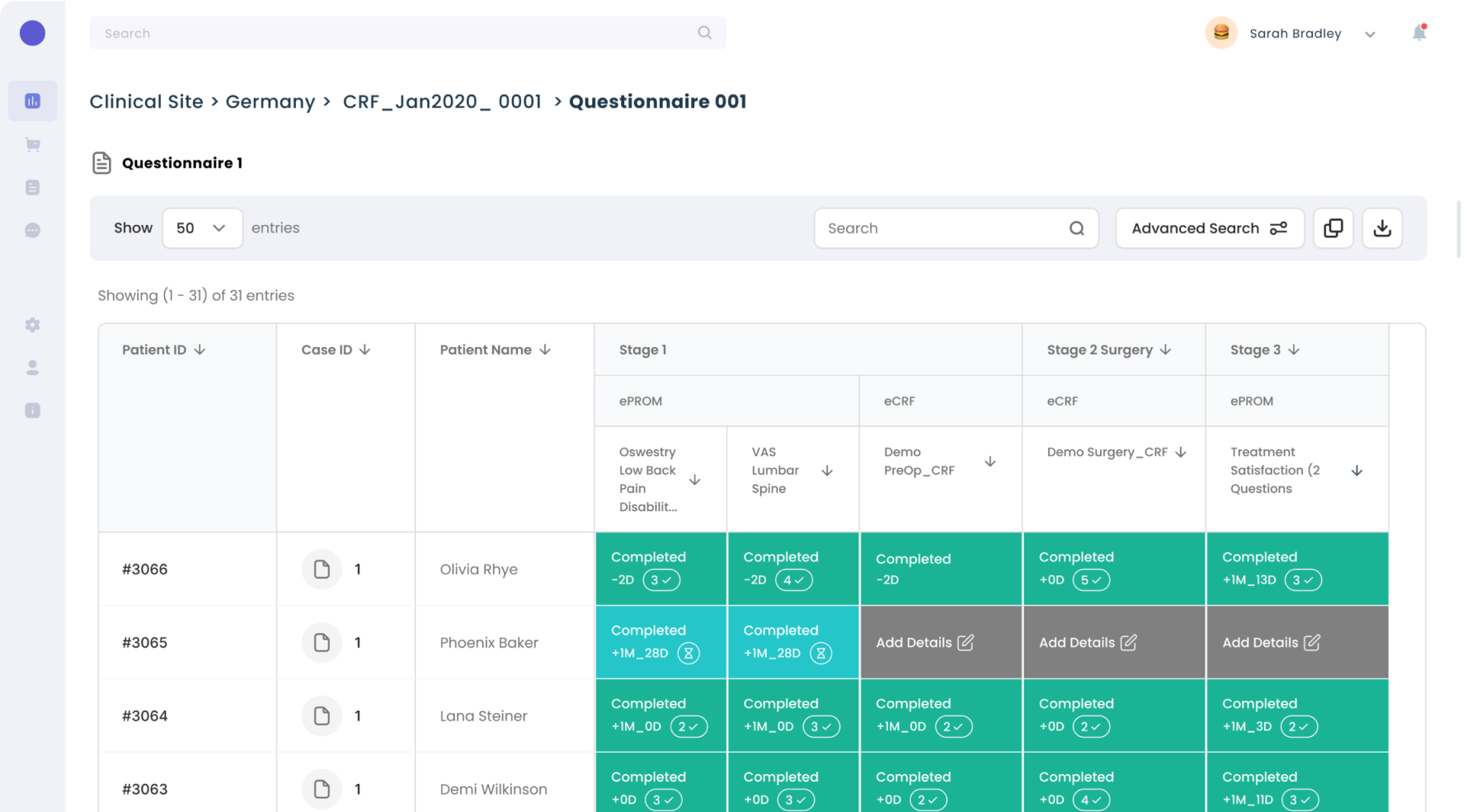
Structural changes to the product for future proofing.
Reduction in time to data discovery
33%
Reduction
Saved hours of manual work
18k+ hrs
Saved
More Case Studies
Your challenges, our mindshare
We believe in focussing on the 40% for delivering 100% impact.
